Long Chain Fatty Acid-Coa Ligase
(All numbering and residues are taken from first PDB file)
![]()
![]()
Bending Residue Dihedral Analysis
Residue
iResidue
i+1Distance of hinge axis to residue i in
(A) Distance of hinge axis to residue i in
(A) Change in
(deg) Change in
(deg) Angle of psi(i) axis to hinge axis
(deg) Angle of psi(i) axis to hinge axis
(deg) Percentage Progress
GLU-429
ILE-430
12.6
12.8
14.6
-5.8
72.2
74.2
2.0
ILE-430
LYS-431
8.9
9.1
-13.3
-10.5
175.4
178.9
-12.6
LYS-431
ASP-432
8.0
8.7
-172.9
-87.6
103.7
113.7
22.5
ASP-432
ARG-433
5.5
5.1
77.2
-72.3
69.8
135.5
2.2
ARG-433
LEU-434
2.9
2.2
136.6
40.7
34.9
75.9
-64.7
LEU-434
LYS-435
1.2
4.7
23.0
4.5
38.4
82.0
15.9
LYS-435
ASP-436
4.3
4.4
146.7
36.0
78.8
28.6
105.4
ASP-436
LEU-437
6.1
5.2
121.5
-61.6
98.0
91.3
18.3
LEU-437
ILE-438
5.9
5.9
25.3
-0.2
55.6
64.8
10.4
ILE-438
LYS-439
7.0
7.1
14.9
-18.8
127.5
135.9
1.6
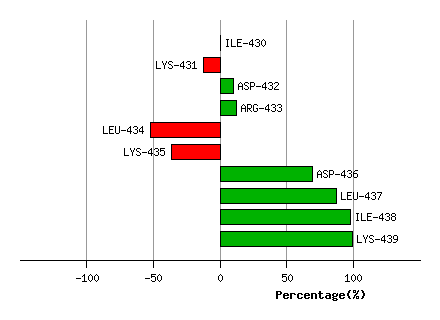
Graph shows rotational transition at bending residues and can be used
to identify hinge bending residues.
Probably only informative for interdomain rotations greater than 20 degrees