Mitogen-Activated Protein Kinase Fus3
(All numbering and residues are taken from first PDB file)
![]()
![]()
Bending Residue Dihedral Analysis
Residue
iResidue
i+1Distance of hinge axis to residue i in
(A) Distance of hinge axis to residue i in
(A) Change in
(deg) Change in
(deg) Angle of psi(i) axis to hinge axis
(deg) Angle of psi(i) axis to hinge axis
(deg) Percentage Progress
LYS-49
PRO-50
12.1
12.1
-58.9
12.1
39.3
52.7
248.2
PRO-50
LEU-51
11.0
11.1
-15.4
-9.8
27.5
15.9
198.0
LEU-51
PHE-52
13.3
13.3
-0.4
0.5
61.2
54.1
-0.2
PHE-52
ALA-53
11.5
11.9
6.8
1.9
109.1
97.3
-34.8
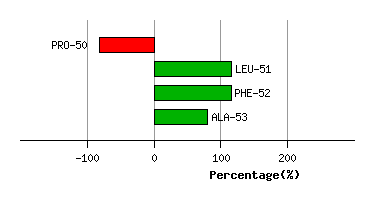
Graph shows rotational transition at bending residues and can be used
to identify hinge bending residues.
Probably only informative for interdomain rotations greater than 20 degrees
Residue
iResidue
i+1Distance of hinge axis to residue i in
(A) Distance of hinge axis to residue i in
(A) Change in
(deg) Change in
(deg) Angle of psi(i) axis to hinge axis
(deg) Angle of psi(i) axis to hinge axis
(deg) Percentage Progress
GLU-330
PHE-331
13.7
13.6
-12.4
6.2
24.0
43.6
26.3
PHE-331
ASP-332
12.1
13.4
-6.0
11.0
81.8
104.4
8.8
ASP-332
HIS-333
14.5
15.8
4.7
2.2
99.0
90.3
9.6
HIS-333
TYR-334
17.5
18.4
-31.0
-31.4
40.5
27.0
456.7
TYR-334
LYS-335
17.4
18.4
5.1
-30.2
124.5
125.4
80.4
LYS-335
GLU-336
20.6
20.1
23.7
48.5
142.3
150.3
-545.7
GLU-336
ALA-337
18.2
18.0
26.0
-17.1
70.3
91.0
40.2
ALA-337
LEU-338
15.5
15.7
9.4
-29.7
108.5
83.8
6.6
LEU-338
THR-339
12.0
12.1
-4.7
-16.8
52.9
41.2
117.2
THR-339
THR-340
9.7
9.6
6.7
-4.7
64.9
69.7
12.9
THR-340
LYS-341
7.0
6.6
-3.4
-0.9
94.2
104.8
-16.2
LYS-341
ASP-342
4.6
4.5
-0.5
2.6
140.7
150.9
10.2
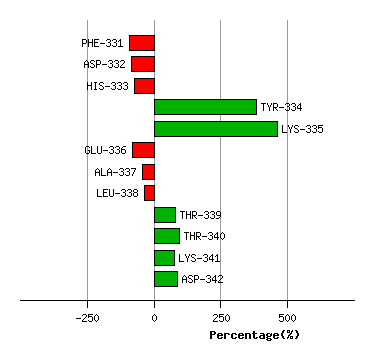
Graph shows rotational transition at bending residues and can be used
to identify hinge bending residues.
Probably only informative for interdomain rotations greater than 20 degrees