Hspbp1 Protein
(All numbering and residues are taken from first PDB file)
![]()
![]()
Bending Residue Dihedral Analysis
Residue
iResidue
i+1Distance of hinge axis to residue i in
(A) Distance of hinge axis to residue i in
(A) Change in
(deg) Change in
(deg) Angle of psi(i) axis to hinge axis
(deg) Angle of psi(i) axis to hinge axis
(deg) Percentage Progress
ARG-237
ALA-238
9.4
9.0
13.5
-24.4
108.6
118.0
44.8
GLN-240
GLN-241
12.9
12.6
26.1
-4.7
156.0
164.3
-101.9
GLN-241
GLN-242
12.3
11.4
-21.4
1.1
90.2
77.0
132.1
GLN-242
VAL-243
14.4
13.7
87.9
-76.3
83.1
98.4
45.5
VAL-243
GLN-244
13.1
12.0
-19.4
15.6
163.6
151.1
-24.6
GLN-244
LYS-245
14.8
14.2
-0.8
-0.5
117.0
121.1
7.4
LYS-245
LEU-246
13.3
11.9
-11.3
14.0
106.4
116.8
-19.1
LEU-246
LYS-247
9.7
8.7
19.0
-20.9
41.1
31.3
-9.7
LYS-247
VAL-248
11.1
11.1
-11.0
13.4
150.1
138.8
9.6
VAL-248
LYS-249
12.2
12.2
1.2
2.4
70.0
70.1
10.1
LYS-249
SER-250
9.6
9.1
-5.8
0.0
109.8
124.6
-30.6
SER-250
ALA-251
6.8
6.9
10.9
-3.8
24.0
21.9
38.6
ALA-251
PHE-252
8.9
9.4
6.3
-13.5
44.3
56.1
-21.2
PHE-252
LEU-253
9.1
9.3
-3.0
1.1
105.5
108.9
-12.6
LEU-253
LEU-254
5.5
5.7
-10.7
11.3
126.6
139.2
16.8
LEU-254
GLN-255
4.8
5.0
4.1
-10.3
21.5
27.2
-21.6
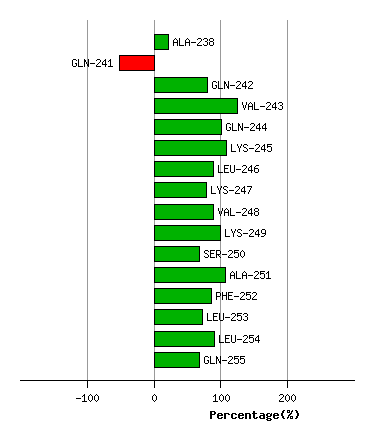
Graph shows rotational transition at bending residues and can be used
to identify hinge bending residues.
Probably only informative for interdomain rotations greater than 20 degrees