Uridylmonophosphate/cytidylmonophosphate Kinase
(All numbering and residues are taken from first PDB file)
![]()
![]()
Bending Residue Dihedral Analysis
Residue
iResidue
i+1Distance of hinge axis to residue i in
(A) Distance of hinge axis to residue i in
(A) Change in
(deg) Change in
(deg) Angle of psi(i) axis to hinge axis
(deg) Angle of psi(i) axis to hinge axis
(deg) Percentage Progress
VAL-123
MET-124
2.6
2.4
-5.6
2.9
112.8
114.3
17.1
MET-124
THR-125
0.7
0.9
-6.1
4.5
144.6
145.6
-12.0
THR-125
GLN-126
1.6
1.2
6.5
-6.1
103.8
100.6
-14.5
GLN-126
ARG-127
4.9
4.6
0.2
-0.5
107.9
108.0
40.0
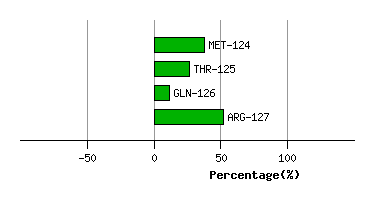
Graph shows rotational transition at bending residues and can be used
to identify hinge bending residues.
Probably only informative for interdomain rotations greater than 20 degrees
Residue
iResidue
i+1Distance of hinge axis to residue i in
(A) Distance of hinge axis to residue i in
(A) Change in
(deg) Change in
(deg) Angle of psi(i) axis to hinge axis
(deg) Angle of psi(i) axis to hinge axis
(deg) Percentage Progress
SER-144
ILE-145
4.5
5.0
4.0
-0.3
30.8
30.0
39.1
ILE-145
LYS-146
3.1
3.4
0.4
-5.0
97.5
91.8
14.8
LYS-146
LYS-147
3.6
3.8
2.2
0.2
111.3
109.7
18.8
LYS-147
ARG-148
6.4
6.7
3.8
3.8
42.0
39.2
38.0
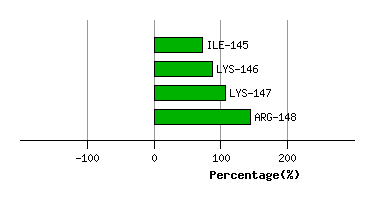
Graph shows rotational transition at bending residues and can be used
to identify hinge bending residues.
Probably only informative for interdomain rotations greater than 20 degrees