Glutamine Binding Protein
(All numbering and residues are taken from first PDB file)
![]()
![]()
Bending Residue Dihedral Analysis
Residue
iResidue
i+1Distance of hinge axis to residue i in
(A) Distance of hinge axis to residue i in
(A) Change in
(deg) Change in
(deg) Angle of psi(i) axis to hinge axis
(deg) Angle of psi(i) axis to hinge axis
(deg) Percentage Progress
LYS-87
SER-88
5.4
4.4
36.0
-46.7
119.0
123.6
9.9
SER-88
GLY-89
1.7
0.7
-6.5
64.8
132.1
129.9
72.3
GLY-89
LEU-90
2.2
2.8
-37.4
32.3
53.1
56.4
-3.2
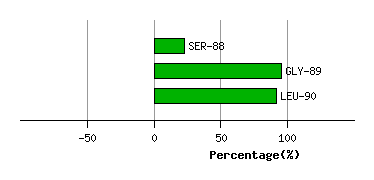
Graph shows rotational transition at bending residues and can be used
to identify hinge bending residues.
Probably only informative for interdomain rotations greater than 20 degrees
Residue
iResidue
i+1Distance of hinge axis to residue i in
(A) Distance of hinge axis to residue i in
(A) Change in
(deg) Change in
(deg) Angle of psi(i) axis to hinge axis
(deg) Angle of psi(i) axis to hinge axis
(deg) Percentage Progress
LEU-180
GLU-181
6.5
7.5
-3.6
-148.2
75.3
65.6
67.6
GLU-181
ALA-182
4.0
4.1
127.4
-10.8
67.1
48.6
115.0
ALA-182
GLN-183
2.1
0.9
5.1
-6.0
114.4
88.3
19.6
GLN-183
GLN-184
2.5
2.6
-35.5
3.8
129.4
113.8
-31.0
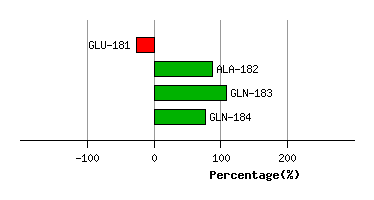
Graph shows rotational transition at bending residues and can be used
to identify hinge bending residues.
Probably only informative for interdomain rotations greater than 20 degrees